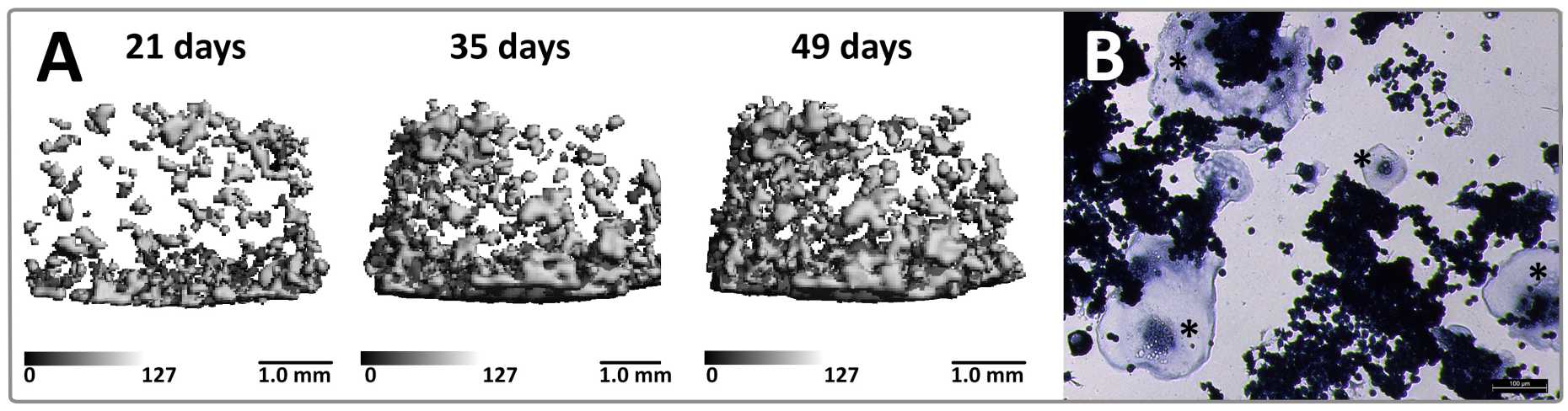
3D Tissue-Engineered Bone Adaptation Model
Bone tissue homeostasis occurs by a coordinated process of bone formation and resorption with various cell types being involved. Communication of bone-forming osteoblasts and bone-resorbing osteoclasts is a fundamental requirement for a balanced bone remodelling. Mechanical stress is an important modulator of cell physiology playing an important role in tissue homeostasis. Osteoblast/osteoclast co-cultures are attractive because they are one step closer to natural conditions and allow elucidation of some aspects of the complex interactions between bone-building and bone-resorbing cells. While several in vitro studies focus on osteoblast/osteoclasts co-cultures in 2D and in 3D models for biomaterial and biomolecule testing, little is known about the cross-talks and cell-cell interactions established in co-culture systems under mechanical stimulation. In addition, in vitro monitoring of the bone adaptation is still missing.
Our research group has designed different bioreactors to exert mechanical stimulation on tissue engineered constructs in order to get an insight of how mechanical loads are transmitted to- and sensed by cells. In addition, these in-house designed bioreactors enable to non-invasively and temporally monitor the development of mineralized extracellular matrix by the use of micro-computed tomography.
Present work focuses on studying bone cell cross-talk in a 3D in vitro environment similar to real bone under mechanical loading. Our goal is to expand the existing technology of non-destructive imaging with mechanical stimulation to a cell based co-culture system. The aim of this model is to provide information on tissue quality and temporal monitoring of bone formation/resorption, either at physiological conditions or in response to the administration of drugs. The co-culture system will be applied i) to identify and characterize bone-like tissue turnover in tissue-engineered constructs, ii) for a better understanding of the reaction of engineered tissue to certain external cues, and iii) to mimic the effects of therapeutic molecules. Ultimately, the system may serve for the identification, characterization and development of new drugs to be used for bone regeneration or to cure bone diseases.