Bone Mechanoregulation in Type II Diabetes
Bone fragility is dependent not only on bone mass but also on microstructure and the intrinsic material properties of the tissue. Although fracture risk is increased in diabetic patients, clinical assessment of bone fragility in type 2 diabetes (T2D) is particularly challenging because patients have an elevated fracture risk despite having normal or even high bone mineral density (BMD). Current clinical diagnostic techniques such as dual-energy x-ray absorptiometry (DXA) consider BMD but not material strength and, therefore, are not able to accurately assess bone fragility in T2D.
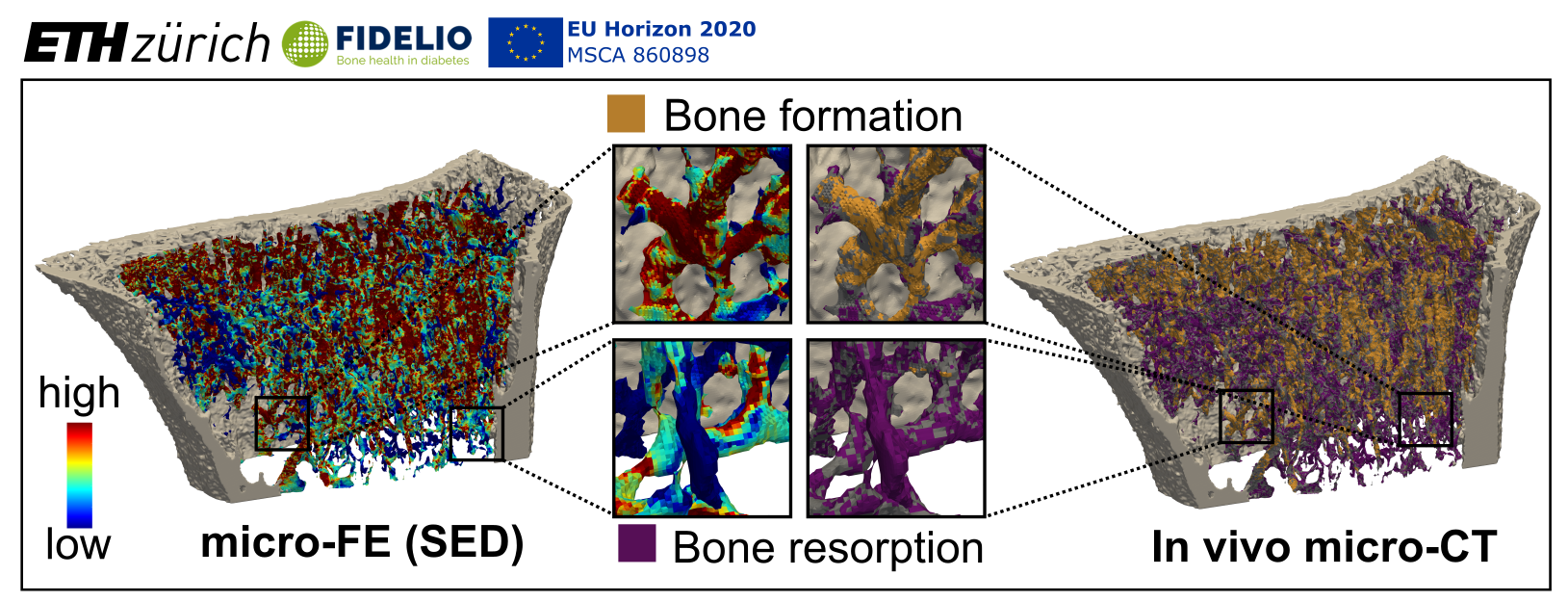
In recent years there has been a shift in focus towards advancing computational models for the characterisation of tissue- and cell-level biomechanical properties of bone. These tools are essential to understand the changes leading to fragility fractures, and to develop new diagnostic approaches to identify diabetic patients at risk of fracture, in a personalised medicine approach. Recent reports stipulate that there is a direct link between diabetes-induced changes in the extracellular matrix (ECM) and changes in cell mechanosensation that may contribute to bone fragility. However, mechanoregulation in diabetic human bone has not been investigated at the local microstructural level thus far. Based on the recent advent of high-resolution peripheral quantitative computed tomography (HR-pQCT), we have developed imaging and computational methods which enable monitoring of local changes in the bone microstructure over time and calculating local mechanical loading. This has been demonstrated in mice and humans at such high spatial resolution that cellular behaviour – in the form of bone remodelling sites – can be studied and the corresponding mechanical loading can be calculated.
There are, however, technological challenges that prevent the use of these advanced imaging and computational methods in clinical studies. Although these computations are fast on supercomputers, they are still too slow and cumbersome to run on local clinical computer systems. In cooperation with IBM, this project aims to push bone imaging and computational methods for mechanobiological bone remodelling studies from bench (supercomputer) to bedside (clinical computing) in the hospital environment. These approaches will then be used to investigate the effects of diabetes on local mechanoregulation of bone remodelling in type 1 diabetes (T1D) and T2D as well as appropriate controls, identifying its relationship to bone fragility and bone biomarkers that are directly linked to this impairment in mechanoregulation.
Project Leader(s):
Matthias Walle, Caitlyn Collins
Collaborators:
Dr. Christiano Malossi, Research Staff Member, Manager AI Automation, Zurich Research Laboratory, Zurich, Switzerland
Prof. Richard Eastell, MD FRCP FRCPI FRCPEdin FRCPath FMedSci, Department of Oncology and Metabolism, Metabolic Bone Centre, Northern General Hospital, Sheffield
For further information on collaborators visit: external pageFIDELIO-project.eucall_made
Acknowledgments:
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860898.