Mechano-Molecular Regulation of Fracture Healing
Fracture healing is regulated by mechanical loading. Understanding the underlying mechanisms during the different healing phases is required for targeted mechanical intervention therapies.
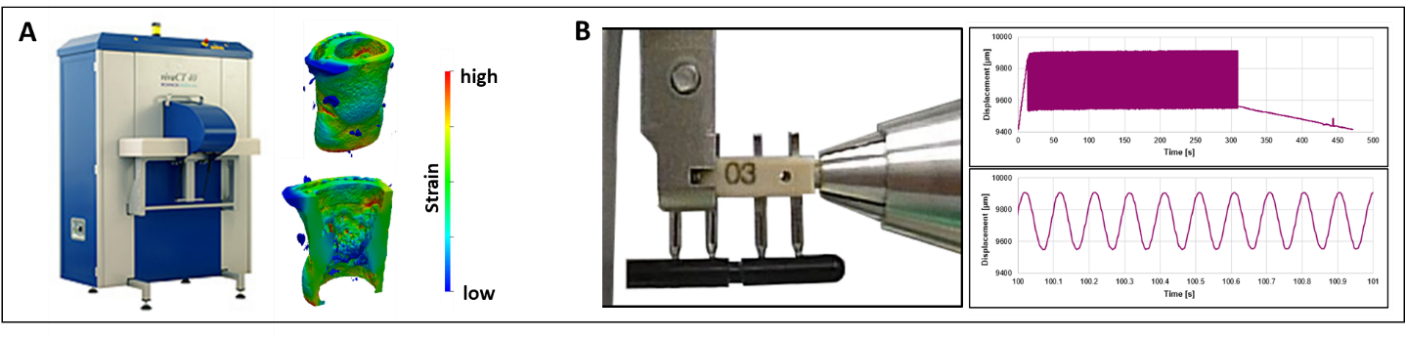
In this project we aimed to overcome current limitations (load not adapted to individual animals, cross-sectional setup, lack of cell type-specific molecular callus analyses) in fracture healing studies by establishing protocols and developing the equipment for individualized cyclic mechanical loading and state-of-the-art in vivo imaging (time-lapsed micro-computed tomography), allowing for spatially-resolved single cell gene expression analysis based on their 3D local in vivo environment (LivE).
To allow for more comprehensive analysis of the healing process, an in vivo micro-CT protocol for longitudinal monitoring of callus formation was developed (Wehrle et al., Sci Rep 2019). To enable precisely controllable local application of cyclic mechanical loading, a novel loading fixator and loading system were designed (Wehrle et al., bioRxiv 2020, Paul et al., bioRxiv 2020). By applying time-lapsed in vivo micro-CT and animal-specific real-time micro-finite element analysis (micro-FEA), the developed femur fracture loading model allows scaling of loading settings based on strain distribution in the callus (Paul et al., bioRxiv 2020). Using this individualized approach, we subsequently showed that the remodeling phase is highly responsive to cyclic mechanical loading with significantly improved callus properties (Wehrle et al., bioRxiv 2020) and distinct effects on Wnt-signalling-associated molecular targets Sclerostin and Rankl in callus sub-regions and the adjacent cortex. The developed equipment and protocols are currently being applied to understand bone healing mechanomics in a mouse model of accelerated aging.
Collaborators
Ariane Scheuren, Duncan Betts, Gisela Kuhn, Graeme Paul, ETH Zurich, Switzerland.
Acknowledgment
We gratefully acknowledge funding from the ETH Zurich Postdoctoral Fellowship Program and from the Marie Curie Actions for People COFUND Program.
We gratefully acknowledge funding from the EU (ERC Advanced MechAGE ERC-2016-ADG-741883). E. Wehrle received funding from the ETH Postdoctoral Fellowship Program (MSCA-COFUND, FEL-25_15-1).
Journal articles
Wehrle, E., Kuhn, G. A., Betts, D. C., Scheuren, A., & Müller, R. (2015). external pageInfluence of Longitudinal In Vivo Micro-Computed Tomography on Fracture Healing in a Femoral Defect Modelcall_made. Tissue Engineering Part A, 21, S406–S406.
Wehrle, E., Liedert, A., Heilmann, A., Wehner, T., Bindl, R., Fischer, L., et al. (2015). external pageThe impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice.call_made Disease Models & Mechanisms, 8(1), 93–104. http://doi.org/10.1242/dmm.018622
Betts, D. C., & Müller, R. (2014). Mechanical Regulation of Bone Regeneration: external pageTheories, Models, and Experiments.call_made Frontiers in Endocrinology, 5(11), e002276. http://doi.org/10.3389/fendo.2014.00211
Trüssel, A., Müller, R., & Webster, D. (2012). external pageToward Mechanical Systems Biology in Bone.call_made Annals of Biomedical Engineering, 40(11), 2475–2487. http://doi.org/10.1007/s10439-012-0594-4