Towards Biomimetic 3D Osteocyte Cultures
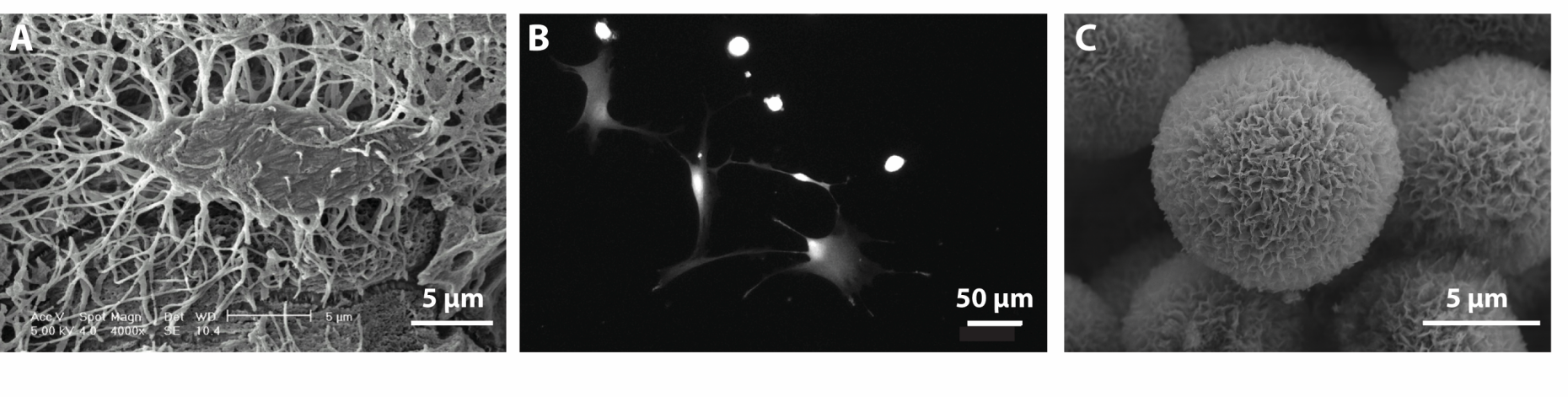
Osteocytes are stellate shaped cells embedded deeply within the matrix of mineralized bone forming a dense, interconnected network of over 20’000 cells per cubic millimeter (A). These cells are widely accepted to sense the mechanical loads that bone is subjected to and to orchestrate the bone remodelling processes that ensue. However, the exact nature of the mechanisms by which osteocytes sense mechanical stimuli and react to them are still unclear. Both their location embedded deeply within the solid, intransparent bone matrix, and their state as terminally differentiated, non-dividing cells have made it very challenging to study this cell type.
The geometry of the cavities in bone that enclose the osteocytes - the lacuno-canalicular network (LCN) - has been predicted by computational models to be important for the transduction of mechanical signal to the cells. Furthermore, LCN structure is altered in bone affected by diseases such as osteopenia, osteoarthritis, osteopetrosis or osteoporosis and in skeletal sites that are subjected to different loads, which also evidences that it has an important influence on osteocytes.
We aim to mimic these geometries and develop a framework to study osteocyte function and dynamics in primary cells (B) as well as cell lines. To reproduce geometries resembling the LCN, state-of-the art micro-3D printing of a transparent, biocompatible material will be used. We further plan to integrate protein and mineral components (C) into the system by means of surface coating in order to emulate the biochemical environment of the osteocytes.