Micro-Multiphysics Modelling of Osteoporosis and its Treatments
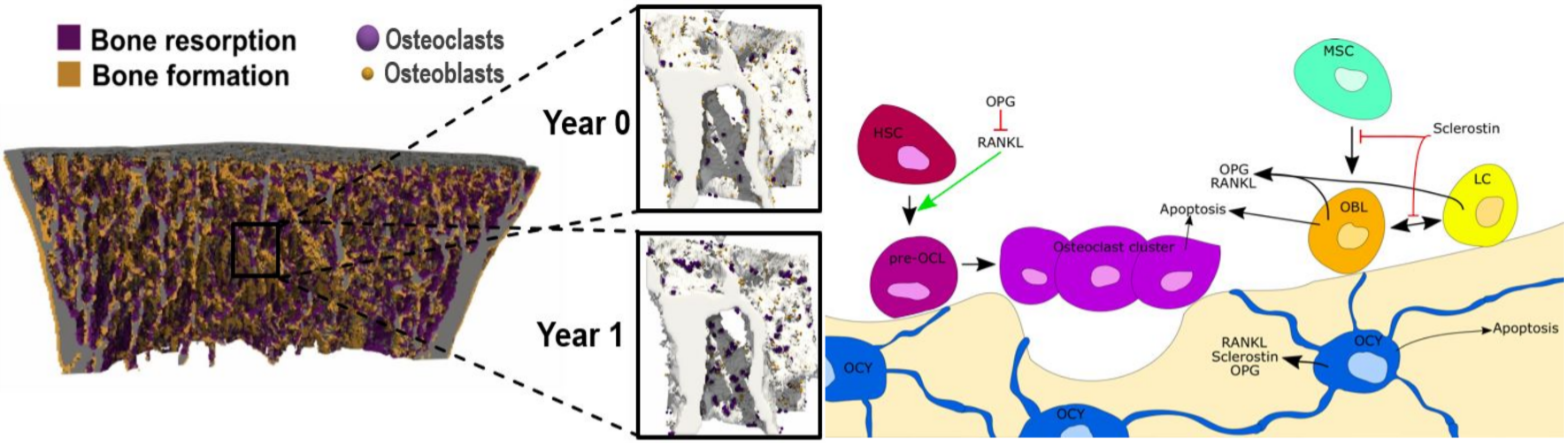
The 9 million osteoporotic fragility fractures that occur every year currently lead to over 400,000 deaths and health care costs in excess of US$80 billion. New strategies for the prevention and treatment of osteoporosis are therefore of the utmost importance. Clinical trials are an essential tool for evaluating treatments and developing further mechanistic understanding of their effect. However, phase 3 clinical trials cost on average 30 million USD over the course of 1 to 4 years; this cost increases by $671,000 with each additional month, meaning that trials on osteoporosis medication lasting up to 10 years are particularly expensive. In silico models could provide a fast and inexpensive tool for testing hypotheses on bone remodeling and for informing clinical trial design; in pre-clinical applications, these models have been shown to make patient-specific predictions of bone degeneration and response to therapeutics. Recent advances in medical imaging have enabled the assessment of in vivo bone microarchitecture at successive time points using high-resolution peripheral quantitative computed tomography (HR-pQCT). To date, these modalities have not been combined.
We developed a novel micro-multiphysics (micro-MP) model that integrates mechanics, cell behavior and reaction-diffusion of signaling molecules and parametrized this model to match bone volume fractions and morphometric measures assessed in clinical trials of denosumab treatment over a 10-year period. Rates of cell differentiation, death, replication, and production as well as reaction and diffusion of signaling molecules in this model were based on literature values from in vivo experimental investigations; if unavailable, in vitro data or parameter fitting were used.
This project aims to evaluate the robustness of the micro-MP model and to integrate longitudinal HR-pQCT patient data for model validation. First, the existing model will be used to simulate denosumab withdrawal from micro-CT scans of iliac crest biopsies. Secondly, the model will be adapted to generate scalable physiologic predictions of bone remodeling based on HR-pQCT data from intact, distal radii of a patient cohort. Upon validation, the model could be used to inform the design of clinical trials and as a method to gain further insights into bone biology and treatments’ method of action.
Project Leader(s):
Charles Ledoux, Caitlyn Collins