Bone Healing Mechanomics in a Mouse Model of Premature Aging
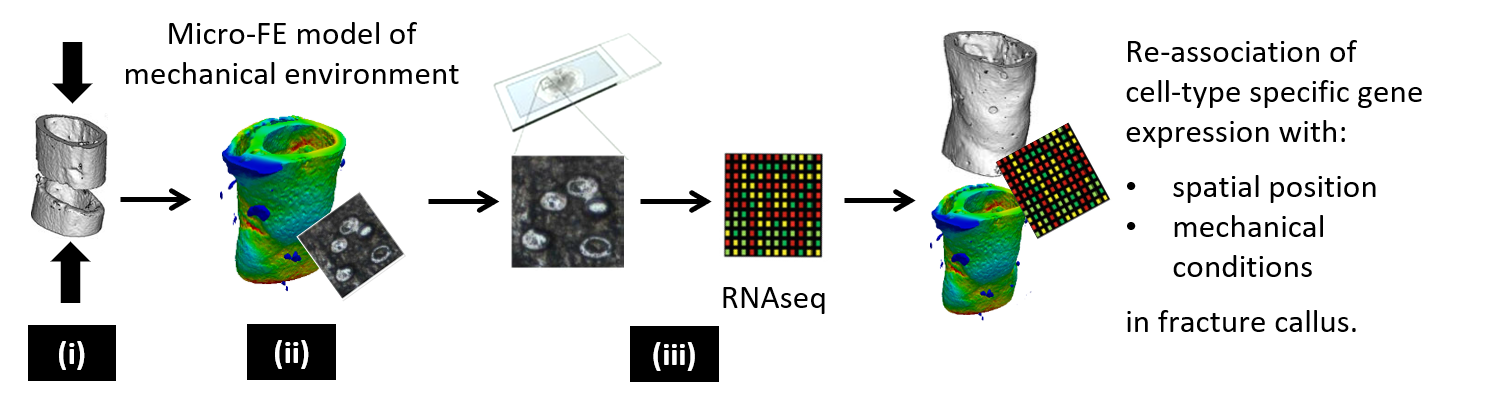
Delayed bone healing and non-unions account for 5 – 10% of all bone fractures and present a challenging problem in regenerative medicine, particularly in elderly osteoporotic patients. Bone healing is a mechano-sensitive process; thus, different mechanical stimuli can either enhance or impair healing. In recent years, the profound influence of the mechanical environment on cell-fate decision and tissue differentiation during bone healing has been recognized. However, the underlying molecular mechanisms by which mechanical stimuli locally alter cellular behaviour are complex and poorly understood. This has prevented wider-scale harnessing of the mechano-sensitivity of the regenerative process in clinical applications. This has also contributed to a lack of consensus on whether bone exhibits age-associated declines in mechano-sensitivity. Understanding these molecular mechanisms of mechanically-regulated tissue formation is therefore crucial to create optimal healing environments for different bone fractures. Our objective is thus to develop a molecular-based understanding of bone healing mechanobiology and to investigate how this mechano-sensitivity is compromised with age. Our approach is to use an established femur defect model in young and aged mice, and apply (i) in vivo imaging (time-lapsed micro-computed tomography, micro-CT), (ii) in silico modelling (micro-finite element analysis, micro-FE) and (iii) spatially-resolved single-cell transcriptomics to quantify how mechanical stimuli are translated by individual cells and subcellular components to form bone at fracture sites.
Furthermore, we will incorporate synchrotron-based imaging techniques into our technological platform to investigate how the local mechanical environment influences the formation of bone at the nanoscale. Bone at the nanoscale consists of collagen fibres and mineral crystals. These constituents of bone together form the various hierarchical structures within bone and contribute to its remarkable strength and toughness. We are thus interested in potential pathways by which these fundamental “building blocks” of bone are formed and assembled in response to local mechanical stimuli in young and aged mice.
Acknowledgements:
We gratefully acknowledge funding from the EU (ERC Advanced MechAGE ERC- 2016-ADG-741883 and MSCA-IF-EF-ST 101029062 - MechanoHealing) and from the SNF (GEMSTONE COST Action IZCOZ0_198152 / 1).