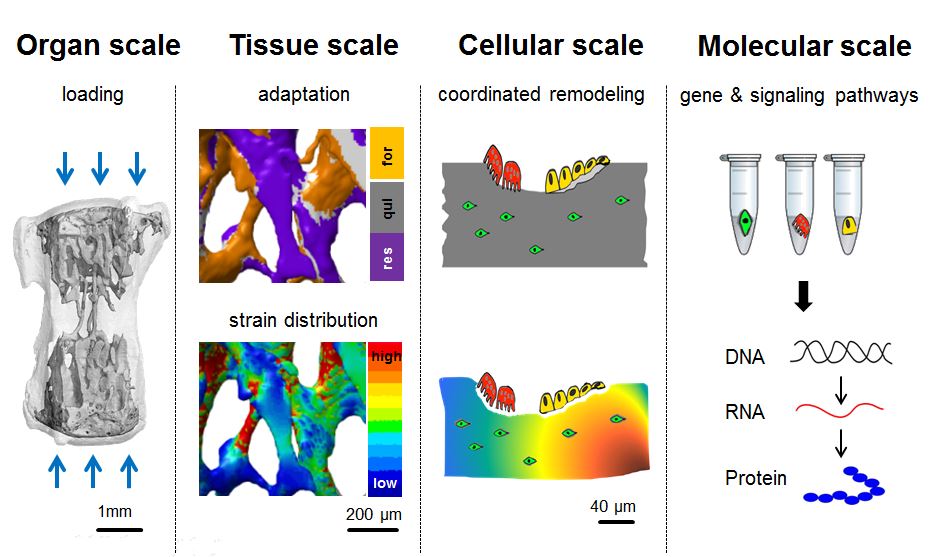
In Vivo Mechanomics
Mechanical loading is an important physiological factor regulating bone adaptation and regeneration. Hence, a detailed understanding of the underlying mechano-molecular mechanisms, also referred to as mechanomics, is needed. This knowledge on mechanomics will allow for identification of mechano-responsive therapeutic targets and enable individualized mechanical intervention therapies for improvement of compromised bone properties and impaired regeneration.
Our Goal
At the in vivo mechanomics team, we aim for a spatio-temporal, systems-level understanding of the mechano-molecular mechanisms governing load-induced bone adaptation and regeneration. We aim to also transfer this mechanomic knowledge to clinical applications.
Our Expertise
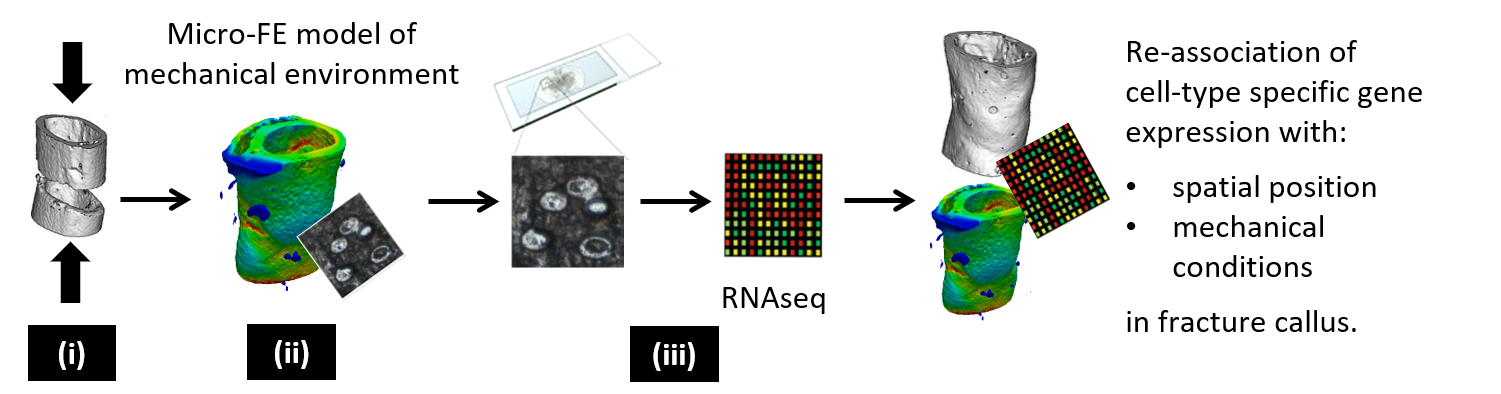
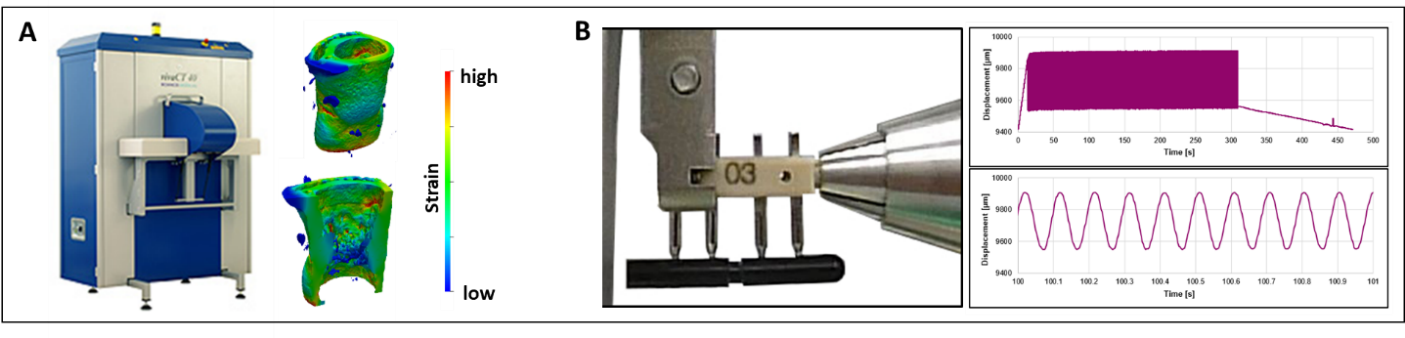
We combine expertise from different disciplines (e.g. biology, engineering, informatics, veterinary medicine) allowing a multi-scale and multi-disciplinary approach combining in vivo models with in silico modelling and omics technologies. We use gene editing for generating mouse lines, which enable the spatio-temporal in vivo assessment of different bone phenotypes during bone adaptation and regeneration. Via application of time-lapsed in vivo micro-computed tomography with subsequent micro-finite element analyses, we want to characterize the local mechanical in vivo environment (LivE) of single bone cells. Omics technologies are then established to investigate molecular responses of cells to LivE changes in well-established and novel mouse models of bone adaptation and regeneration. Based on the in vivo findings observed, we develop novel in silico models and computational tools, allowing prediction of individualized bone adaptation and regeneration capacities under different diseases and treatments.
Projects
Multiscale Modelling of Bone Adaptation and Healing
Bone Healing Mechanomics in Aging
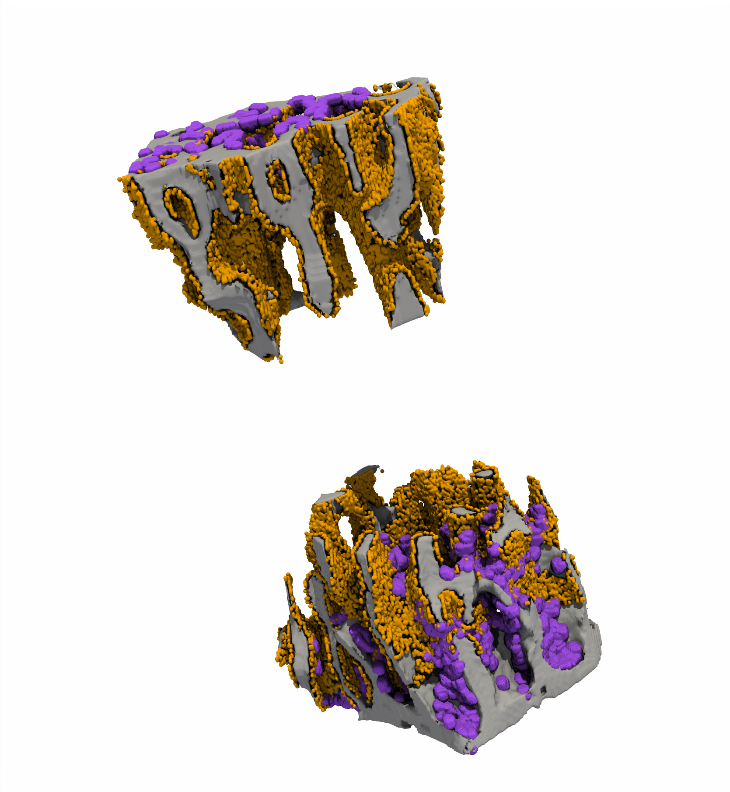
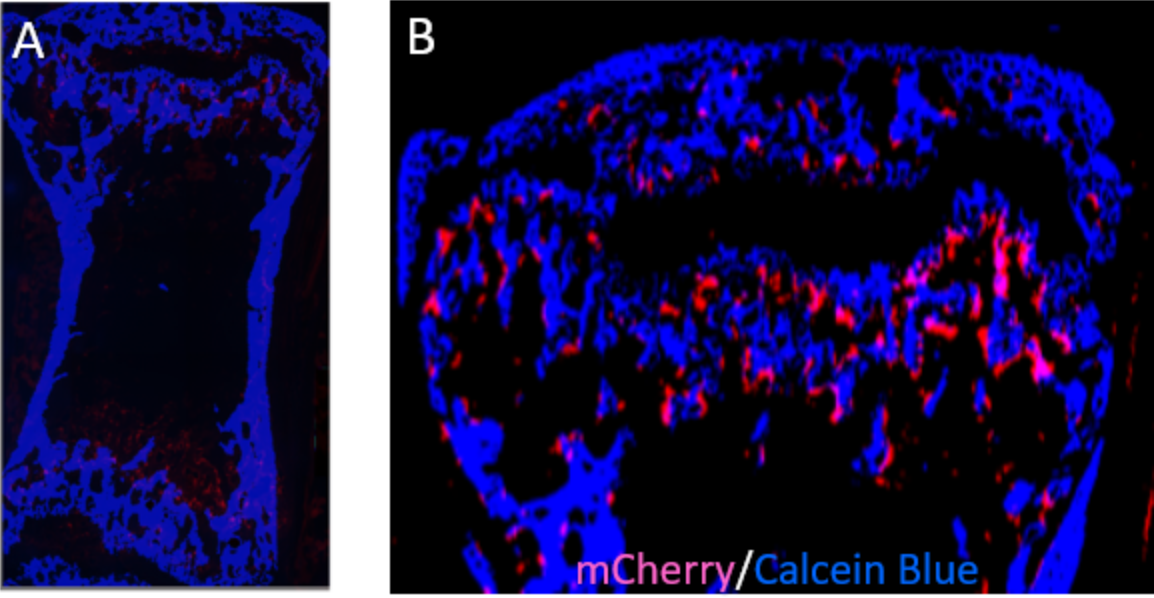
CRISPR/Cas9-Based Fluorescent Reporter Mice for Osteoblasts and Osteoclasts to Study Bone Mechanomics
Osteoporosis is one of the most degenerative diseases that result in a reduction in bone mass and increased fracture risk and partly contribute to the decrease in mechanical usage of the skeleton. By employing CRISPR/Cas9 genome editing, we recently developed fluorescent reporter mice to identify osteoclasts and osteoblasts, which are involved in the bone remodeling process, and help to better understand bone mechanomics.
Mechano-Molecular Regulation of Fracture Healing
Spatial Genomics of Muscle-Bone Interactions during Bone Regeneration
The importance of muscle-bone interactions in bone regeneration has been observed for decades. However, impairment of these interactions under age-induced osteosarcopenic conditions and their implications for regeneration have yet to be fully elucidated. Improving our understanding of the role of muscle in bone regeneration will therefore provide new opportunities to develop therapies to augment the regenerative response.
In Vivo Single-Cell Transcriptomics in Bone
By combining RNA sequencing of single cells isolated by laser-capture microdissection with micro-finite element analysis based on time-lapsed in vivo micro-computed tomography, we propose a systems level approach to allow spatially resolved transcriptomic profiling of individual cells from mechanically loaded bone.