Micro-Multiphysics Modelling of Osteoporosis and its Treatments
With over 10 million fragility fractures annually and healthcare costs soaring, innovative solutions for bone fragility are critical. Here, we developed a micro-multiphysics agent-based (micro-MPA) model to simulate bone remodeling under osteoporosis treatments. Leveraging advanced CT imaging and clinical data, we aim to create predictive tools for personalized therapies, optimize drug regimens, and provide insights into unexplained clinical phenomena like treatment rebound effects.
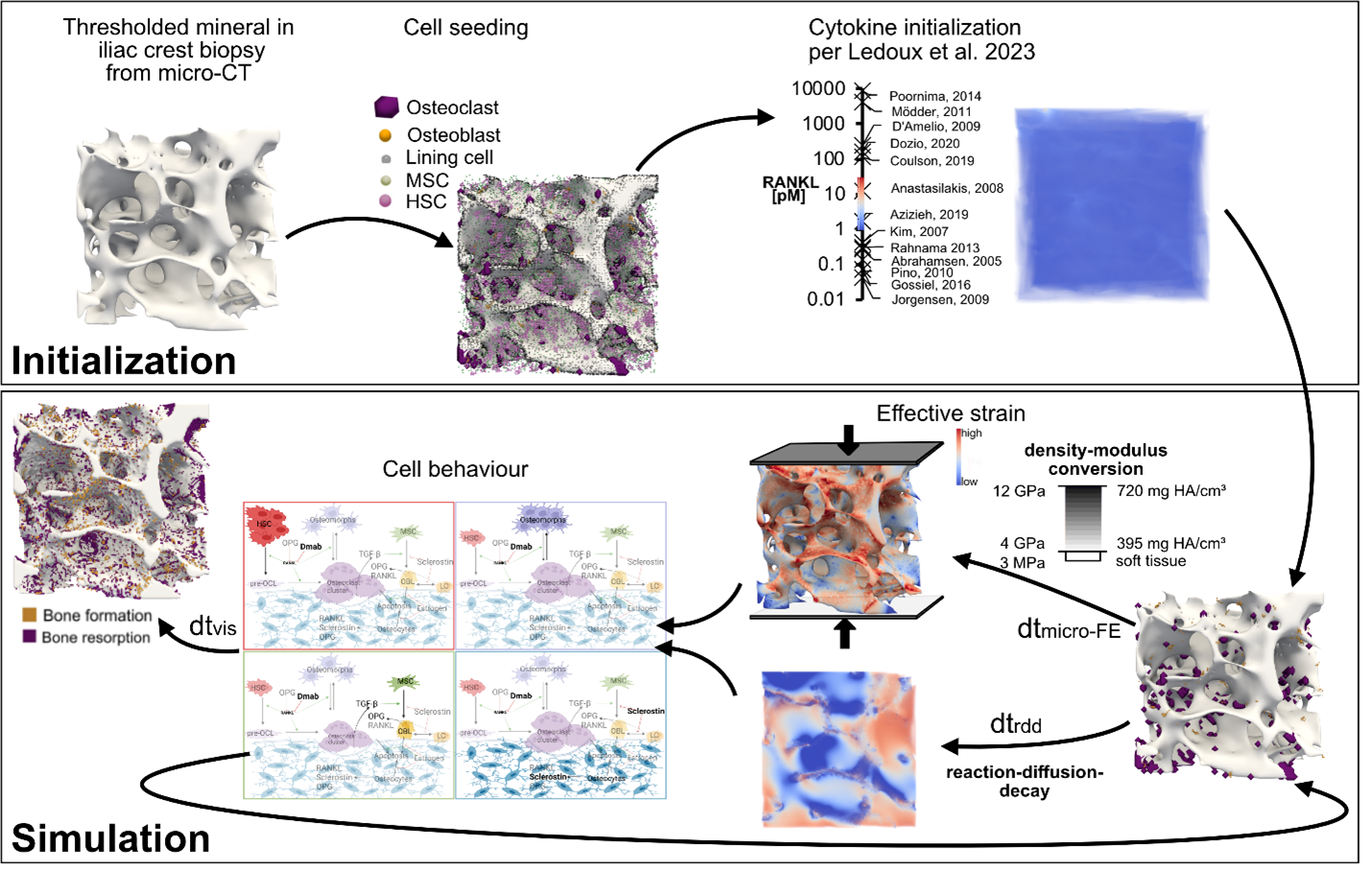
As the global population increases and average age rises, the silver tsunami overwhelms various facets of our healthcare systems including a rapid rise in the number of age-related bone fragility fractures. More than 10 million fragility fractures occur every year worldwide that lead to over 400,000 deaths. New strategies for the prevention and treatment of bone fragility are therefore of the utmost importance. Clinical trials are an essential tool for evaluating treatments and developing further mechanistic understanding of their effect. However, phase 3 clinical trials cost on average 30 million USD over the course of 1 to 4 years; this cost increases by $671,000 with each additional month, meaning that trials on osteoporosis medication lasting up to 10 years are particularly expensive. In silico models could provide a fast and inexpensive tool for testing hypotheses on bone remodeling and for informing clinical trial design; in pre-clinical applications, these models have been shown to make patient-specific predictions of bone degeneration and response to therapeutics.
At the Laboratory for Bone Biomechanics, we created a novel in-house micro-multiphysics agent-based (micro-MPA) model capable of generating physiologic simulations of the local changes in mechanics, cell behavior and reaction-diffusion of signaling molecules in trabecular bone undergoing bone loss due to post-menopausal osteoporosis. Rates of cell differentiation, death, replication, and production as well as reaction and diffusion of signaling molecules in this model were based on literature values from in vivo experimental investigations; if unavailable, in vitro data or parameter fitting were used.
Starting from a representative selection of iliac crest patient biopsies scanned with micro-computed tomography (micro-CT), our model simulates treatment with anti-catabolic (denosumab, bisphosphonates) and anabolic (romosozumab, teriparatide) drugs with static and dynamic morphometric changes that correspond to those reported in clinical trials. We aim to create a library of simulations and a forecast tool for the prediction of morphometric changes with different drug doses, treatment timings, combinatorial and sequential drug treatments. We also aim to quantify the relative contributions of different cellular pathways towards well-known but unexplained clinical phenomena such as the rebound effect observed after denosumab discontinuation or the dual-action effect of romosozumab.
Recent advances in medical imaging have enabled the assessment of in vivo bone microarchitecture in humans at successive time points using high-resolution peripheral quantitative computed tomography (HR-pQCT). We aim to create a personalized model capable of matching follow-up in vivo scans using baseline scans, treatment type, dosing and timing, patient age and gender, and bone turnover marker measurements.
Collaborators
Prof. Dr. Caitlyn Collins, Virginia Tech, Blacksburg, USA
Prof. Dr. Kurt Lippuner, Bern University Hospital, Bern, Switzerland
Contact
Institut für Biomechanik
Gloriastrasse 37/ 39
8092
Zürich
Switzerland
