CRISPR/Cas9-Based Fluorescent Reporter Mice for Osteoblasts and Osteoclasts to Study Bone Mechanomics
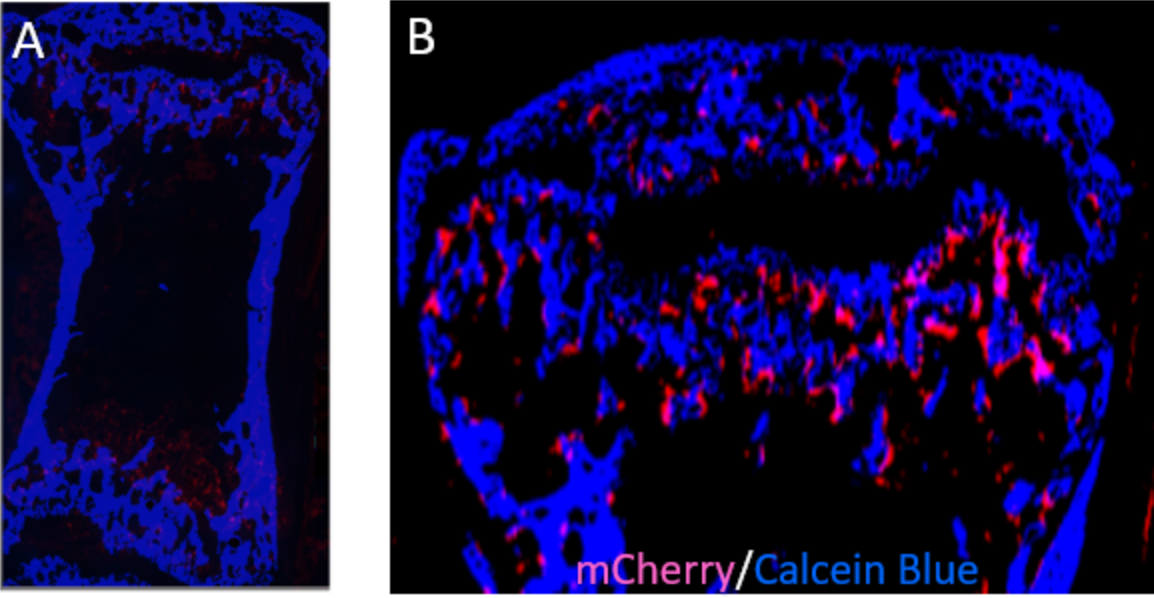
Osteoporosis is one of the most degenerative diseases that result in a reduction in bone mass and increased fracture risk and partly contribute to the decrease in mechanical usage of the skeleton. Therefore, understanding the molecular mechanisms underlying load-regulated bone remodeling can help us to identify molecular targets that can be used to target novel therapies. Bone remodeling is a process of complex interactions between multiple bone cells and their local 3D environments. However, the underlying mechanisms of how cells respond to mechanical signals are still unclear. By employing CRISPR/Cas9 genome editing, our lab recently developed fluorescent reporter mice to identify osteoclasts and osteoblasts which are involved in the bone remodeling process. By combining RNA sequencing of single cells isolated by laser-capture microdissection with micro-finite element analysis and time-lapsed in vivo micro-CT, we will track them dynamically in mouse bone and investigate the molecular response to changes in mechanics in their 3D local in vivo environment (LivE) which will allow us to investigate molecular responses of the cells to their LivE environment and further it will be combined with mouse models of advanced aging, previously characterized in our lab. Thus, the dual-fluorescent reporter mouse lines combined with a prematurely aging mouse model along with the RNA sequencing approaches will allow to rapidly identify osteoblasts and osteoclasts samples from in vivo experiments (tail loading, femur defect loading) for spatially resolved single-cell mechanomics during bone adaptation and regeneration with aging.
Related projects:
Acknowledgements:
We gratefully acknowledge funding from the EU (ERC Advanced MechAGE ERC-2016-ADG-741883).